|
I.
Surface
Modification of Steels History of the usage of
stainless steel has existed for so many years. In our lab, hot-dip galvannealed
sheet steel is mainly focused. Hot-dip galcannealed sheet steel is a widely utilized source for
automotive body panels and parts due to its excellent corrosion resistance
properties. Although the alloying
process and lubrication during working improves the formability of the sheet
steel, for large and more complex parts, a more developed lubricant film
coating is required during molding and working process. In addition, the
Fe-Al interfacial layer is also considered to have some effects. |
|
Surface Coating on Advanced High Strength Steels
|
When the pre-coating
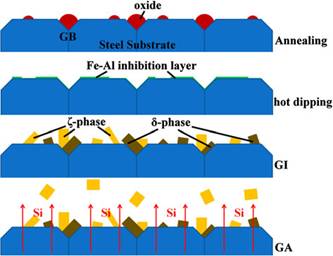
annealing process was conducted at an extremely low dew point, the external selective
oxidation prevailed, resulting in various oxides on the steel surface.
However, the oxides did not fully cover the steel surface under the reduction
atmosphere. Therefore, the molten Zn(Al) reacted
locally with the steel surface to form Fe-Al inhibition alloys. Moreover, the
Al in the molten Zn(Al) bath effectively removed the
oxides by aluminothermic reduction. This Fe-Al
inhibition alloy was then gradually transformed to Al-bearing Fe-Zn phases.
During the following GA process, the diffusion of Si from the steel substrate
to the coating apparently suppressed the forming of the ζ
phase, in which the Si is almost indissoluble. As a result, the Fe-Zn alloy
formation was largely retarded; specifically, a discontinuous Fe-Zn IMCs
layer was formed after 25 seconds of galvannealing
at 773 K (500 ℃). |
|
|
II.
Surface
Modification of Titanium-based Alloys Due to the truth of titanium
and its alloys have excellent biocompatibility with bone tissues,
they are well-selected as implant materials. In this case, a calcium- and
phosphorus-containing film on titanium had been proved to further enhance its
bioactivity after being implanted in human body. We recently focused on the microarc oxidation process to have the surface of titanium be modified. Besides, the SBF immersion process were found to be able to increase the contents of calcium and phosphorus in the anodic film. |
|
|
III.
Surface
Modification of Magnesium-based Alloys Magnesium alloys have become a
group of the most widely used materials for their outstanding mechanical
properties. However, the poor corrosion resistance is a critical weakness of
most magnesium alloys. In improving their resistance to corrosion, surface
modification is an indispensable process. Among various modification
treatments, conversion coating
and anodic oxidation are the most commonly used methods. In the conversion coating for the
magnesium alloys, we have worked for several years on various systems,
including chromate, phosphate-permanganate, and rare-earth metals. Beside the
improvement, our researches especially focus on the microstructure and the
mechanism, which are still less disscussed now. |
|
Conversion Coating on Mg Alloys
|
The formation
mechanism of conversion coating on magnesium alloys is a topic of both
thermodynamics and kinetics.
Through various characterizations, a further understanding of the
relations among microstructure, compositions, and electrochemical properties
may be acquired, which is of great help in developing conversion coating
systems providing sufficient corrosion resistance and mechanical properties. |
|
Permanganate Conversion Coating on AM30 Magnesium
Alloys
|
The hexavalent chromate conversion coating (CCC) developed by
the Dow chemical company had been widely adopt to improve the corrosion
resistance of magnesium alloys. However, hexavalent
chromium is harmful to human health. Consequently, replacing traditional
chromate conversion coating with a new developed conversion process becomes
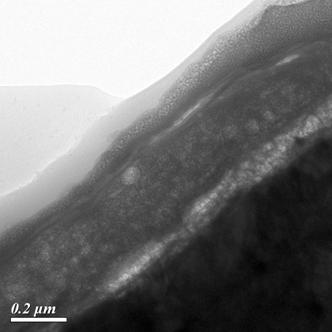
increasingly essential. The TEM
image showed that the thickness of permanganate coating is approximately 200
nm, and the coating is mainly composed of manganese dioxide with an amorphous
structure. In short, the permanganate conversion coating showed better
adhesion and less severe crack by virtue of increasing the chemical
reactivity and
lowering the immersion time, expecting to replace hexavalent chromate conversion coating and reduce the
cost of production. |
|
Cerium Conversion Coating on AZ91 Magnesium Alloys
|
Cerium
conversion coatings were made on AZ91 magnesium plates in cerium nitrate
aqueous solution with H2O2. H2O2
addition increases the reaction rate; however, the blisters on the conversion
coating cause poor adhesion and deteriorate the corrosion resistance of the
cerium conversion coating. An alternative approach by the addition of sodium metavanadate (NaVO3) was used to solve the
formation of blisters. Microstructure observation shows that complex
precipitates are formed on the coating and the amount of blister is
significantly reduced. The results suggest that the presence of VO3-
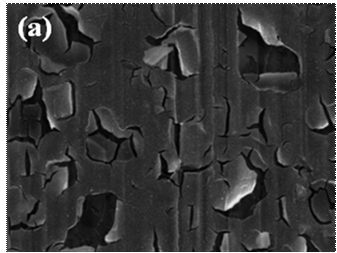
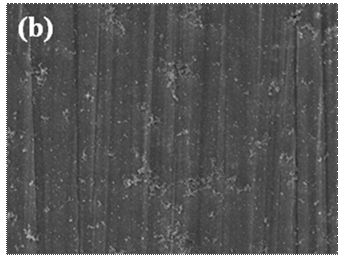
plays an important role in inhibiting the formation of blisters. (Figure.(a)-(b)
Surface morphology of the cerium conversion coating with/without NaVO3,
immersion time 20s; (a) many blisters remains on the cerium conversion
coating without NaVO3 and (b) some precipitates forms on the
coating whereas the blisters are eliminated effectively.) |
|
IV. Electrochemical deposition
|
Electrochemical
deposition is a kind of chemical reaction which has been investigated for a
long run, and the process applies to lots of fields and industries, such as the
automobile industry, cellular phone industry and anti-corrosion field. In
electrochemical process, the main idea is applying an electric field, driving
the cations in electrolyte to be reduced on the
cathode. All the electrochemical reactions happen on the surface of
electrode, hence also regarding as a type of surface science. By means of
method, we can get the material we want or modify the surface of the
electrode. |
|
CIS/CIGS solar cell
|
Cu(InGa)Se2-based
solar cells have often been touted as being among the most promising of solar
cell technologies for cost-effective power generation. This is partly due to
the advantages of thin films for low-cost, high-rate semiconductor deposition
over large areas using layers only a few microns thick and for fabrication of
monolithically interconnected modules. Perhaps more importantly, very high
efficiencies have been demonstrated with Cu(InGa)Se2 at both the cell and the module
levels. Currently, the highest solar cell efficiency is 19.2% with 0.5 cm2
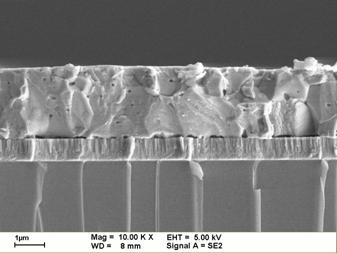
total area fabricated by the National Renewable Energy Laboratory (NREL). The
picture above show the plan view and cross-sectional images of Cu(InGa)Se2-based
solar cells which fabricated by the National Renewable Energy Laboratory
(NREL). A compact grain structure is observed in cross-section, and faceted
grains are visible in plan view. |
|
V.
Sol-Gel
process
|
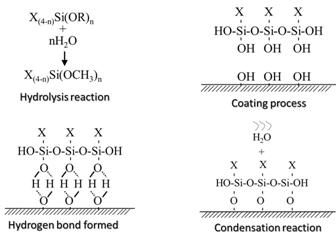
Sol-gel process Is a method
for producing thin films or solid materials. The mechanism of sol-gel process
is the combinative chemical reaction of hydrolysis and condensation. The
precursor such as alkylsiloxane would be hydrolyzed
to the silanol by adding deionized
water in proper pH value. Then the silanol was
self-condensation by removing water. The products formed to the silica
finally. The surface morphology or surface chemical activity could be
modified by sol-gel process. It is widely used in powder metallurgy,
ferroelectrics, superconducting material, ceramic material,
thin film, etc. |
|
Sol-gel coating on the HDG steels
|
Zinc
coating provided the barrier protection and the sacrificial protection over
the steel substrate. To further protect the Zn-coated steel against corrosion
during its transportation and storage, surface modification is generally
adopted. The sol gel coating process was involved the hydrolysis reaction and
the condensation reaction of the precursor. This study is aim to produce a
functional and environmental friendly coating with an excellent corrosion
protection performance on the zinc substrate using a sol-gel process |
|
| Faculty | Research | Teaching | Members | Downloads | Links | Home |
2007
Surface Modification Laboratory. All Right Reserved.
Website administrator: Daniel MT Chen
Website updated by Z.K. Chen